Insights Articles
Articles

November 7, 2024
We invite you to join this KOL webinar featuring two globally recognized U.S. pulmonology experts to get their views on the data from REALITY and how eyonis™ LCS may impact lung cancer diagnostics and treatment practices in the real world. Panelists include Prof. Anil Vachani, from the Hospital of the University of Pennsylvania, and Prof. Javier Zulueta, from the Icahn School of Medicine at Mt Sinai. Fredrik Brag, CEO of Median Technologies, also will participate and summarize the final REALITY results, which are going to be submitted in H1 2025 to regulators for U.S. and EU marketing authorizations for eyonis™ LCS.
Insights Articles

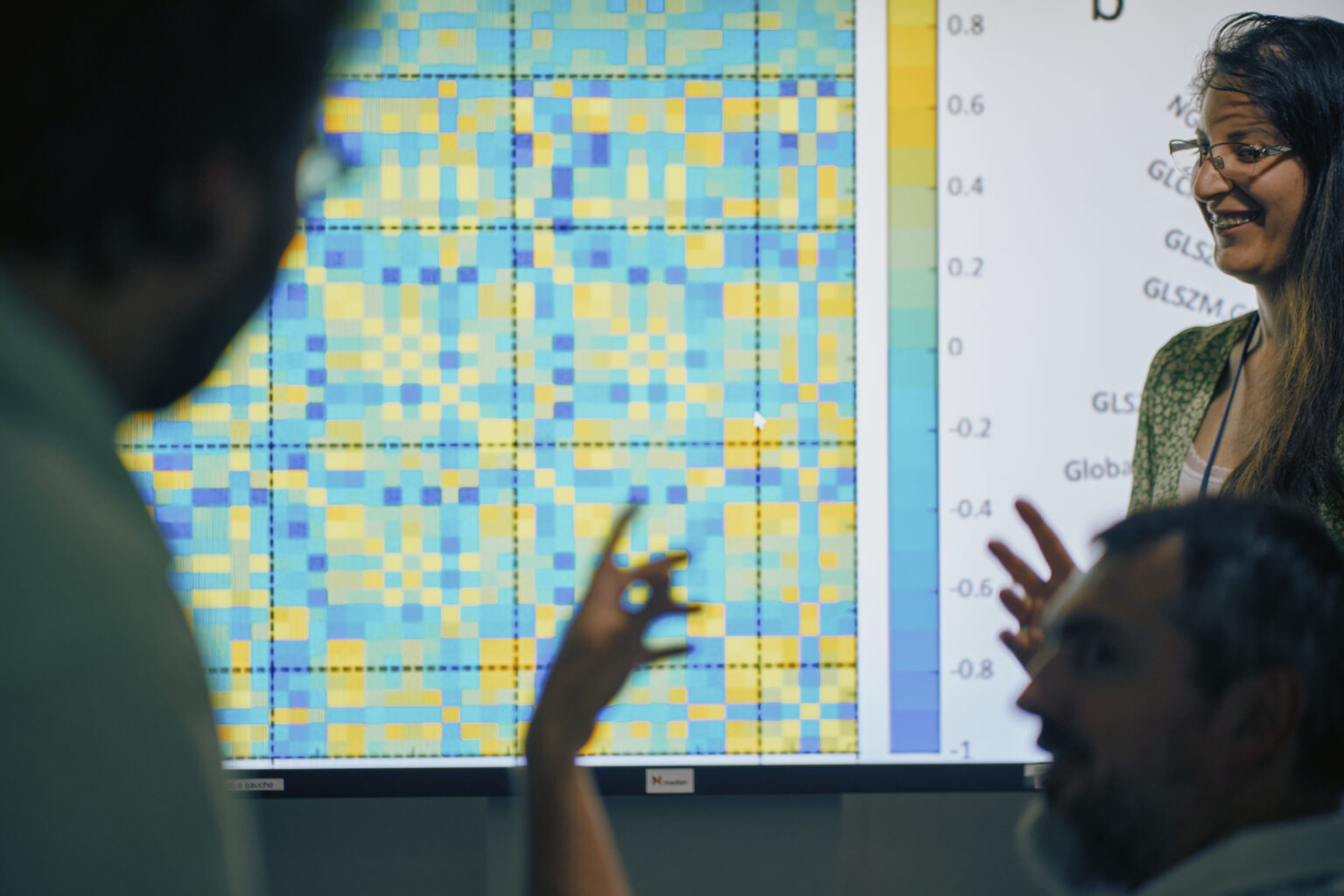
We recently recorded a conversation with Fadila Zerka, Clinical Research Scientist at Median Technologies. Hosted by Connor Anderson, U.S. Project Manager II at Median Technologies, the discussion was about the transformative potential of radiomics in medical imaging and clinical trials. Fadila is an expert in this field with six years of experience. Her research interests are centered around the application of Radiomics in AI, to build new, innovative imaging biomarkers. In this podcast episode, Fadila shared her insights on how this cutting-edge technology is revolutionizing the way we approach diagnosis and treatment planning. They delved into the intricate process of […]
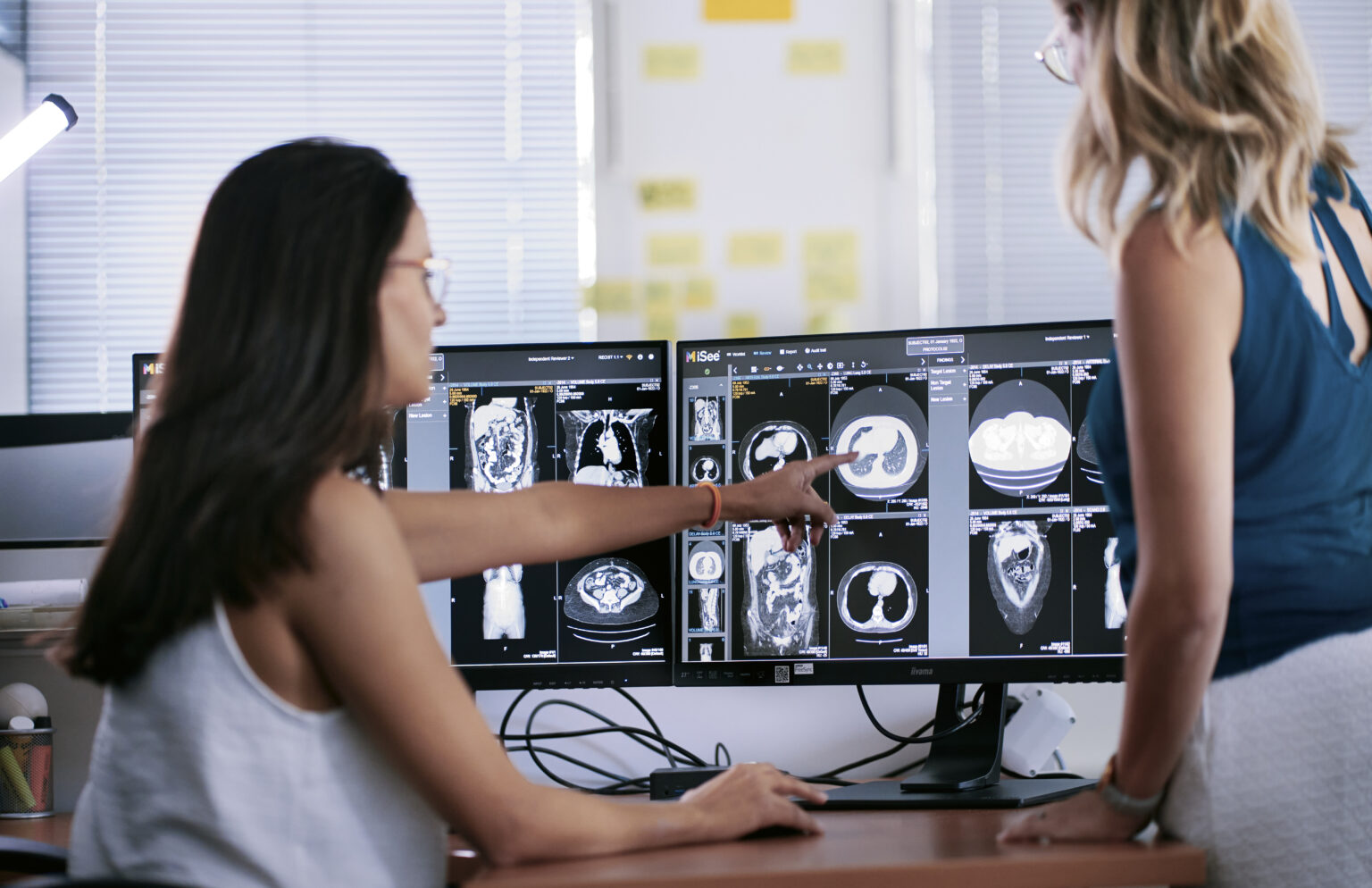
This is an excerpt from our podcast “Shaping Results through Imaging: Addressing Variability in Oncology Trials”. Listen to full version of the podcast here : Connor Anderson – Project Manager II, Median Technologies: Thank you for joining this podcast. We are going to start the interview by setting the stage. Can you describe the role imaging variability plays in oncology drug trials and how it can impact trial results? Dr. Antoine Iannessi, Head of Medical Affairs, Median Technologies: Currently, imaging plays a crucial role in assessing the efficacy of oncology drugs. Simply put, imaging allows us to visualize tumor size, progression, […]

This enlightening discussion delves into how artificial intelligence (AI) can advance oncology clinical trials, ultimately leading to improved care and outcomes for patients, guided by sponsors committed to pioneering advancements in oncology clinical trials. Learn more about:• Advantages of AI-powered imaging for sponsors and patients, using lung cancer as the framework for discussion• Finding early-stage patients for oncology clinical trials using AI-powered patient data and insights• And much more about AI’s potential to impact how we leverage imaging data for the advancement of cancer research. Listen now to our podcast: The Competitive Advantages Of AI-powered Clinical Trial Imaging to Advance […]
In this article, written by AI Imaging expert and Head of Imaging Lab at Median, Sebastien Jacques, we explore 5 incredible ways imaging AI is reshaping the landscape of imaging in oncology clinical trials. 1. Enhanced Image Analysis Traditionally, medical images acquired during clinical trials are interpreted by human experts only. Given the amount of subjectivity involved in the current process, image analysis done this way is at risk of variability. With the emergence of imaging AI, this landscape has drastically changed. AI-powered algorithms can rapidly and accurately analyze vast amounts of medical imaging data, providing quantitative measurements and objective […]
In clinical trials using imaging, biomarkers and response criteria are used to assess the tumor evolution with therapy. We have often heard these terms used interchangeably but the distinction between the two is important in a clinical trial with imaging. Imaging biomarkers are often root contributors to a clinical trial’s endpoints, but they are not the same.

The Value of Standardized Quality and Performance Metrics in Clinical Trials It’s not just tumor measurements that matter! Quality and Performance Measurements are critical too. Imaging biomarker metrics are not the only measurements that matter in oncology imaging trials. Quality and performance metrics are just as important and necessary for running a successful clinical trial with image analysis metrics as the endpoints. Biopharma organizations optimize clinical trials by operationalizing both quality and performance metrics. The need for the industry to adopt vetted, standardized operational time, cost and quality metrics for tracking and predicting performance is clear. Metrics should have standard […]